Sputnik V vaccine trials begin in India
02 Dec 2020 08:46:59
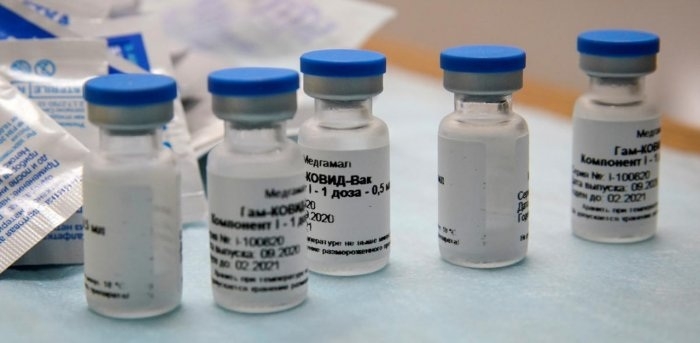
HYDERABAD:
DR REDDY’S Laboratories Ltd. and Russian Direct Investment Fund (RDIF) on Tuesday announced commencement of adaptive phase 2/3 clinical trials for COVID-19 vaccine Sputnik V in India after receiving necessary clearance from the Central Drugs Laboratory, Kasauli. The clinical trials are being conducted by JSS Medical Research as the clinical research partner.
Meanwhile, vaccine development company Serum Institute, India said the Covidshield vaccine will not be released for mass use unless it is proven immunogenic, and safe. The company also said the serious adverse event that happened to a city based volunteer was in no way induced by the vaccine.